| |
| Names | |
|---|---|
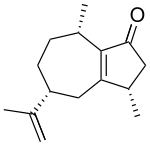
| IUPAC name
Guaia-1(5),11-dien-2-one
| |
| Systematic IUPAC name
(3S,5R,8S)-3,8-Dimethyl-5-(prop-1-en-2-yl)-3,4,5,6,7,8-hexahydroazulen-1(2H)-one | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C15H22O | |
| Molar mass | 218.340 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Rotundone is a sesquiterpene originally discovered in the tubers of Java grass (Cyperus rotundus). Rotundone is also present in the essential oils of black pepper, marjoram, oregano, rosemary, basil, thyme, and geranium, as well as in some Syrah wines.[1][2] It imparts a peppery aroma.[3]
References
- ^ Siebert, Tracey E.; Wood, Claudia; Elsey, Gordon M.; Pollnitz, Alan P. (2008). "Determination of Rotundone, the Pepper Aroma Impact Compound, in Grapes and Wine". Journal of Agricultural and Food Chemistry. 56 (10): 3745–8. doi:10.1021/jf800184t. PMID 18461962.
- ^ "Overlooked pepper compound spices up red wine". Royal Society of Chemistry.
- ^ "Rotundone Imparts Peppery Aroma". Chemical & Engineering News.

